The following 30-second video
shows how our technology works
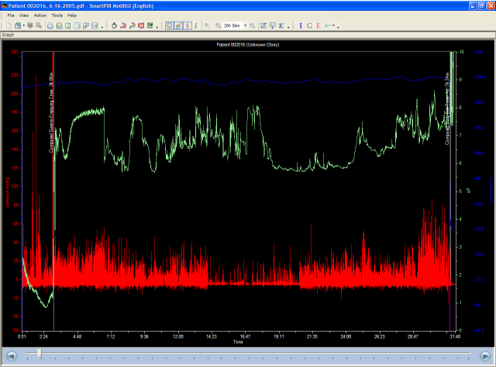
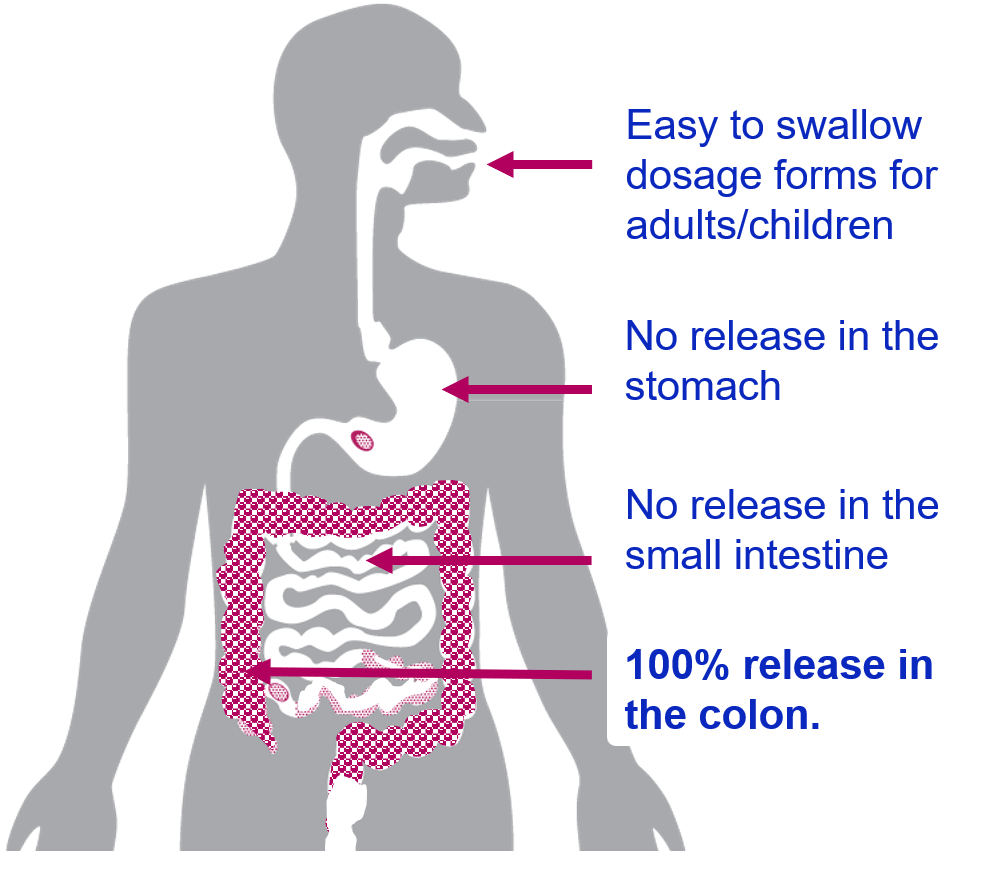
GEMICEL® Technology Can Deliver 100% to the Colon
Only GEMICEL works in tandem with colon-specific pH and pressure
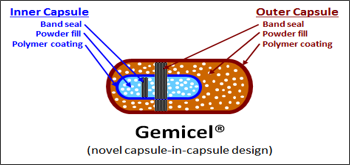
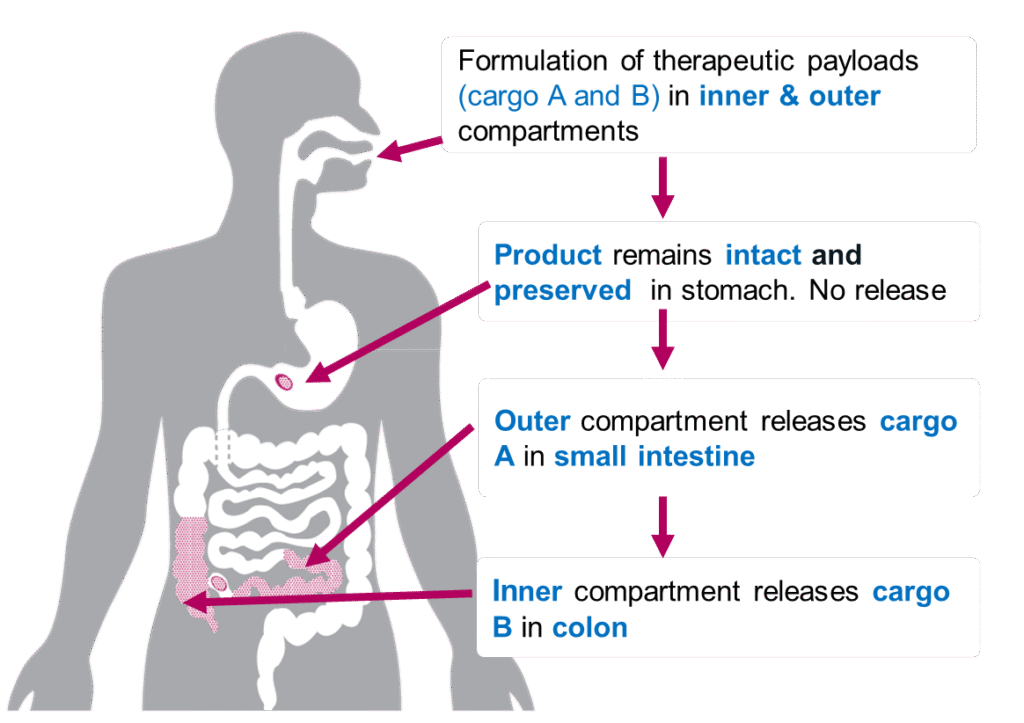
GEMICEL® can also deliver to targeted areas of both the small
intestine and the colon


Intellectual Property Strategy
Opportunity to Extend Patent Life and Exclusivity
- US: 5 issued (9,907,755, 10,369,111, 11,590,083, 10,588,857 & 11,622,936)
- Ex-US:19 issued (available upon request)
- Invented by Mohan Kabadi and Jerome Schentag
- Wholly owned by TheraBiome, LLC
Opportunity for extending patent (up to +20 years):
- An existing drug or biologic
- A compound with a new indication
- A compound that is clinically superior to 1st gen.
- Combination of known compounds
Know-How and Trade Secrets
- Manufacturing

Differentiated Drug Delivery Target Product Profile (TPP)
- TheraBiome’s GEMICEL® platform technology is Phase II-ready and based on its strong science and human proof-of-concept clinical studies compliant with FDA and international regulatory standards.
- The platform is suitable for targeted oral delivery of small molecules and biologics, including vaccines to the colon or to both small intestine and colon.
- Longer acting and locally delivered
- TheraBiome is ready to support CMC strategy and deliverables
- Disease and therapeutic area agnostic despite special focus on GI-related diseases.
- Easy to swallow – patient compliant products
Phase II-Ready Precision GI Targeting
Unmet Medical Need: Oral Targeted Enteral Delivery
Targeted oral delivery technologies include conventional pH-sensitive enteric coatings, multi-matrix, osmotic-controlled and timed-release methods. Limitations include variable or off-target delivery, reduced potency, leakage from one site to another, lack of patent protection and manufacturing complexity, as well as high COGS. GEMICEL overcomes these challenges cost-effectively based on its single- or dual-targeted drug delivery platform. This patented delivery technology is based on dual polymer layers programmed to dissolve at specific pH and peristaltic pressure levels. GEMICEL in various oral forms delivers drugs and biologics to specific target locations in the GI tract. This technology is perfectly designed to target the microbiome with products which otherwise cannot achieve their intended effects. As an innovation it effectively addresses unmet medical needs across multiple-therapeutic areas.
Scintigraphy studies were conducted in 27 healthy volunteers. GRAS ingredients available and scale-up completed. Phase I and IB studies were initiated in 24 subjects before Abbvie ended the ulcerative colitis study following AbbVie’s acquisition of Allergan. Human clinical scintigraphy studies confirmed that GEMICEL is highly tunable and reliably delivers high therapeutic payloads.
