TheraBiome, LLC
TheraBiome has created groundbreaking drug delivery technology: GEMICEL® enables reliableand precise drug delivery to the colon, or to the small intestine and colon.
- This patented technology is Phase II ready. Scintigraphy studies were conducted in 27 healthy subjects for proof-of-concept. Phase IB (Assembly and Allergan) studies were initiated in 24 patients.
- The GEMICEL platform enables new and revamped therapeutics, is differentiated and provides access to an extended product life cycle.
- TheraBiome is keenly interested in exploring R&D collaborations and partnerships across multiple indications.
Our Team has Extensive R&D and Commercialization Expertise
Mohan Kabadi, PhD
Founder, Managing Partner




Jerome Schentag, PharmD
Co-founder, Partner



Michael Atkin
Partnership Advisor





Amit Jolly, BD Advisor
Carpe Biosciences Family Office


Our Team has Extensive Experience and Success in Partnering GEMICEL
- 2013: TheraBiome, LLC founded. Grants exclusive license to Assembly Bio
- 2015: Assembly completes clinical validation in a human scintigraphy
- 2017: Allergan paid $50m up front to Assembly for 6 GI products ($2.7bn)
- 2019: FDA clears an IND for UC and a Phase 1b clinical study is initiated
- 2021: AbbVie acquires Allergan. TheraBiome retains worldwide IP rights

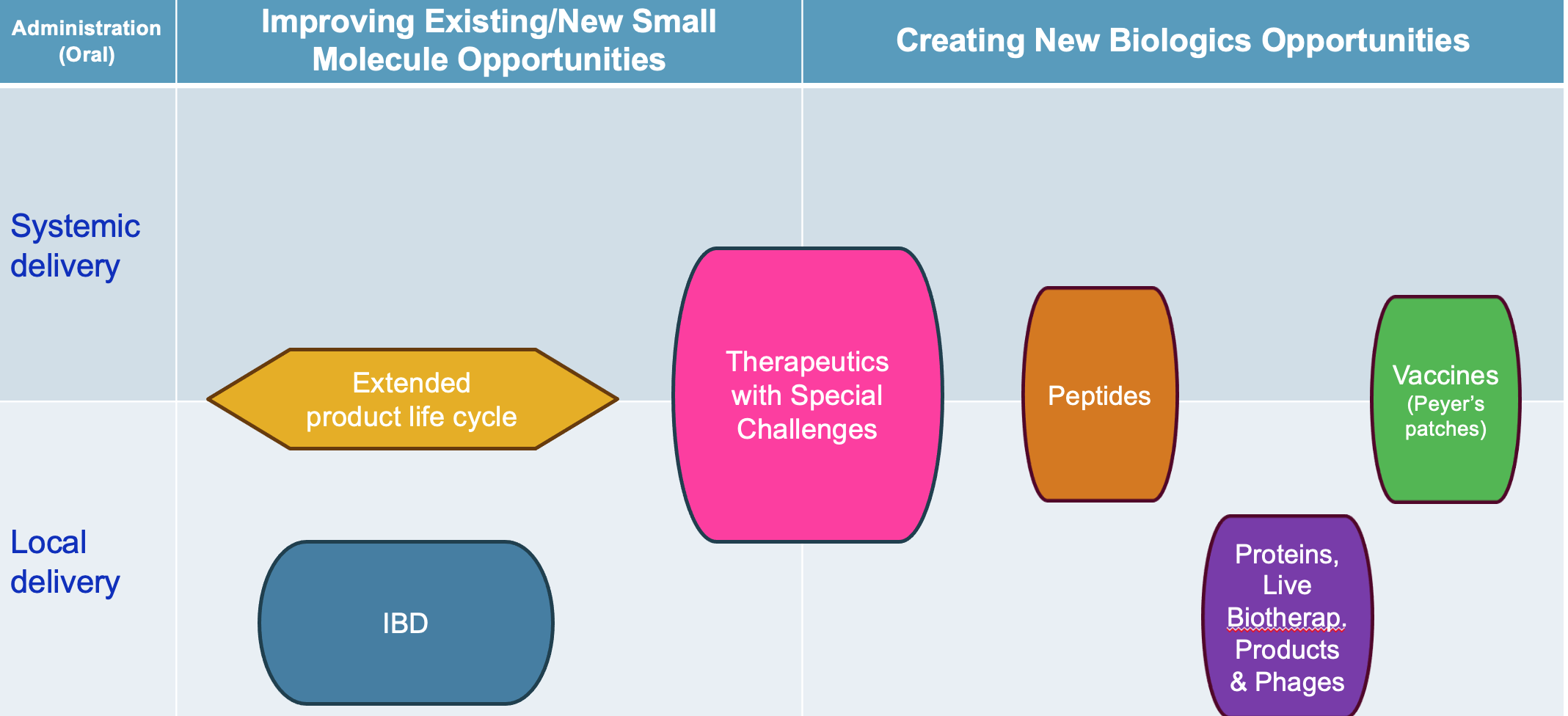
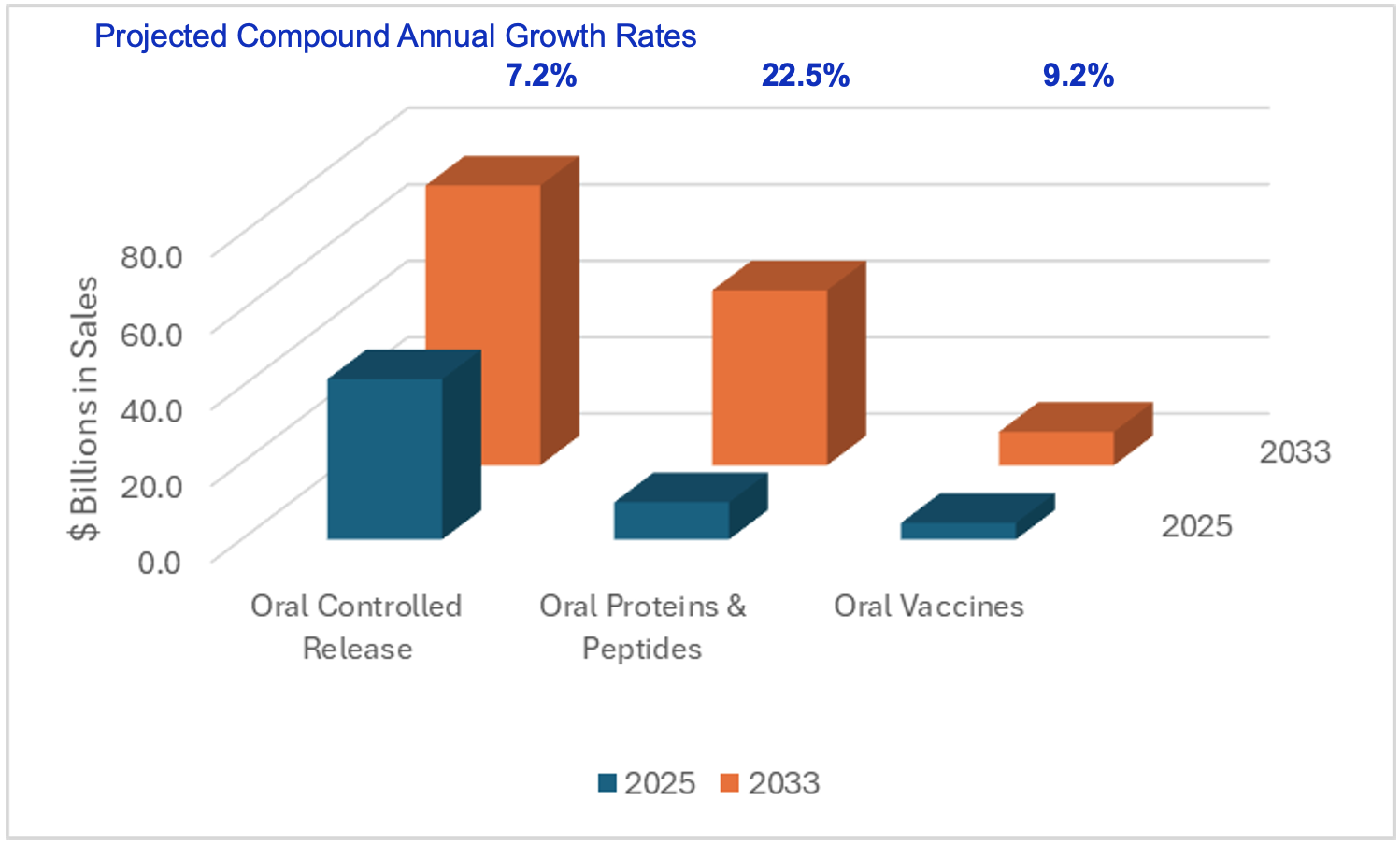
Market Opportunity for GEMICEL
2025-33 Targeted Oral Delivery Market ($ Bns)
GEMICEL’s Go-To-Market Strategy
Solutions to
medical needs
medical needs
Science-driven platform. Enabling customized delivery. Drugs and biologics.
Disease agnostic. Easy to swallow dosages. Lowers pill burden.
Disease agnostic. Easy to swallow dosages. Lowers pill burden.
Market
Potential
Potential
Patented platform technology validated (with data).
Differentiated. Solves a clear unmet need. Potential for Life Cycle Management (LCM)
Differentiated. Solves a clear unmet need. Potential for Life Cycle Management (LCM)
Lower
Risks
Risks
Commercially viable. Cost-effective manufacturing.
Globally compliant, safe excipients (GRAS)
Globally compliant, safe excipients (GRAS)
Path
Forward
Forward
Gemicel is Phase 1/2 clinical trial ready. Maximizes alignment with TPP.
Experienced team ready to partner.
Experienced team ready to partner.
TheraBiome IP, Experience, Goals
- TheraBiome is A Leader in Targeted GI Drug Delivery Technology
- Private New Jersey–based company founded in 2013 by two managing partners
- TheraBiome holds 5 issued US patents for targeted GI tract drug (9,907,755, 10,369,111 & 11,590,083) and vaccine delivery (10,588,857 & 11,622,936). 19 issued patents in other countries
- Patent Counsel: Gearhart Law LLC
- The TheraBiome management team has decades of experience in drug delivery engineering and biotech. partnering, as well as PK/PD expertise
- This colon- or dual-targeted technology enhances therapeutic efficacy while minimizing side effects of small molecules, peptides, proteins, live biotherapeutic products (LBP), and vaccines through controlled-release formulations. GEMICEL overcomes limitations of conventional enteric coatings, facilitates the developability of new or revamping of failed drugs, is differentiated and provides access to an extended product life cycle management.
- Human scintigraphy confirms reliable payload delivery to specific sites within the GI tract through pH- and pressure-dependent release mechanisms.
- TheraBiome builds on the Assembly Bio/ Allergan collaboration which advanced GEMICEL technology to the clinic for treating inflammatory bowel disease (IBD), e.g., ulcerative colitis and Crohn’s disease.
- This technology holds promise in Inflammatory Bowel Disease (IBD), e.g., Crohn’s and ulcerative colitis and enables oral administration for local and systemic applications. GEMICEL is differentiated and provides access to renewed product vigor with extended life cycle management.
- Partnering goals: licensing, co-development, joint venture, acquisition etc.
- Ready to provide consulting support and knowhow for drug product and technology transfer